Need Help?
800-229-7252
Lab Chemicals

-

-

-

-
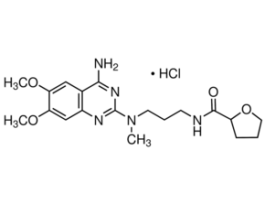 Alfuzosin for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0000832SIGY0000832-EA
Alfuzosin for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0000832SIGY0000832-EA -

-
 Alfuzosin hydrochloridepharmaceutical secondary standard; traceable to USP and PhEurPHR1638SIGPHR1638-1G-EA
Alfuzosin hydrochloridepharmaceutical secondary standard; traceable to USP and PhEurPHR1638SIGPHR1638-1G-EA -
 Alfuzosin hydrochlorideUnited States Pharmacopeia (USP) Reference Standard1012917SIG1012917-150MG-EA
Alfuzosin hydrochlorideUnited States Pharmacopeia (USP) Reference Standard1012917SIG1012917-150MG-EA -
 Alfuzosin System Suitability Mixture AUnited States Pharmacopeia (USP) Reference Standard1012871SIG1012871-25MG-EA
Alfuzosin System Suitability Mixture AUnited States Pharmacopeia (USP) Reference Standard1012871SIG1012871-25MG-EA -
 Alfuzosin System Suitability MixtureUnited States Pharmacopeia (USP) Reference Standard1012928SIG1012928-15MG-EA
Alfuzosin System Suitability MixtureUnited States Pharmacopeia (USP) Reference Standard1012928SIG1012928-15MG-EA -
 Alimemazine for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0001581SIGY0001581-EA
Alimemazine for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0001581SIGY0001581-EA -

-
 Aliphatics Mix (C5-C12)certified reference material, 2000 mug/mL each component in methanolUST-157SIGUST157-1.5ML-EA
Aliphatics Mix (C5-C12)certified reference material, 2000 mug/mL each component in methanolUST-157SIGUST157-1.5ML-EA -

-
 Alkalinity, CaCO3 1000 mg/L Calibration Standardcertified reference material 100MLALK1000-100SIGALK1000-100ML-EA
Alkalinity, CaCO3 1000 mg/L Calibration Standardcertified reference material 100MLALK1000-100SIGALK1000-100ML-EA -
 Alkalinity, CaCO3 1000 mg/L Calibration Standardcertified reference material 500MLALK1000-100SIGALK1000-500ML-EA
Alkalinity, CaCO3 1000 mg/L Calibration Standardcertified reference material 500MLALK1000-100SIGALK1000-500ML-EA -
 Alkalinity, CaCO3 500 mg/L Calibration Standardcertified reference materialALK500-100SIGALK500-500ML-EA
Alkalinity, CaCO3 500 mg/L Calibration Standardcertified reference materialALK500-100SIGALK500-500ML-EA -
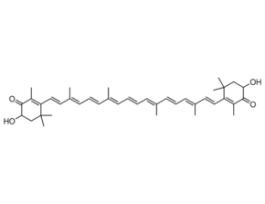
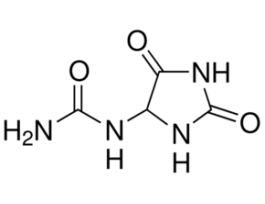
-

-

-

-

-

-

-

-

-

-
 Allopurinol Related Compound AUnited States Pharmacopeia (USP) Reference Standard1013024SIG1013024-25MG-EA
Allopurinol Related Compound AUnited States Pharmacopeia (USP) Reference Standard1013024SIG1013024-25MG-EA -
 Allopurinol Related Compound BUnited States Pharmacopeia (USP) Reference Standard1013013SIG1013013-25MG-EA
Allopurinol Related Compound BUnited States Pharmacopeia (USP) Reference Standard1013013SIG1013013-25MG-EA -
 Allopurinol Related Compound CUnited States Pharmacopeia (USP) Reference Standard1013035SIG1013035-25MG-EA
Allopurinol Related Compound CUnited States Pharmacopeia (USP) Reference Standard1013035SIG1013035-25MG-EA -
 Allopurinol Related Compound DUnited States Pharmacopeia (USP) Reference Standard1013046SIG1013046-35MG-EA
Allopurinol Related Compound DUnited States Pharmacopeia (USP) Reference Standard1013046SIG1013046-35MG-EA -
 Allopurinol Related Compound EUnited States Pharmacopeia (USP) Reference Standard1013068SIG1013068-25MG-EA
Allopurinol Related Compound EUnited States Pharmacopeia (USP) Reference Standard1013068SIG1013068-25MG-EA -
 Allopurinol Related Compound FUnited States Pharmacopeia (USP) Reference Standard1013079SIG1013079-25MG-EA
Allopurinol Related Compound FUnited States Pharmacopeia (USP) Reference Standard1013079SIG1013079-25MG-EA