Need Help?
800-229-7252
Lab Chemicals

-
-

-

-
 Citalopram for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0000855SIGY0000855-EA
Citalopram for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0000855SIGY0000855-EA -

-
 Citalopram hydrobromideUnited States Pharmacopeia (USP) Reference Standard1134233SIG1134233-200MG-EA
Citalopram hydrobromideUnited States Pharmacopeia (USP) Reference Standard1134233SIG1134233-200MG-EA -

-
 Citalopram Related Compound AUnited States Pharmacopeia (USP) Reference Standard1134244SIG1134244-25MG-EA
Citalopram Related Compound AUnited States Pharmacopeia (USP) Reference Standard1134244SIG1134244-25MG-EA -
 Citalopram Related Compound BUnited States Pharmacopeia (USP) Reference Standard1134255SIG1134255-25MG-EA
Citalopram Related Compound BUnited States Pharmacopeia (USP) Reference Standard1134255SIG1134255-25MG-EA -
 Citalopram Related Compound CUnited States Pharmacopeia (USP) Reference Standard1134266SIG1134266-25MG-EA
Citalopram Related Compound CUnited States Pharmacopeia (USP) Reference Standard1134266SIG1134266-25MG-EA -
 Citalopram Related Compound DUnited States Pharmacopeia (USP) Reference Standard1134277SIG1134277-15MG-EA
Citalopram Related Compound DUnited States Pharmacopeia (USP) Reference Standard1134277SIG1134277-15MG-EA -
 Citalopram Related Compound EUnited States Pharmacopeia (USP) Reference Standard1134288SIG1134288-25MG-EA
Citalopram Related Compound EUnited States Pharmacopeia (USP) Reference Standard1134288SIG1134288-25MG-EA -
 Citalopram Related Compound FUnited States Pharmacopeia (USP) Reference Standard1134299SIG1134299-200MG-EA
Citalopram Related Compound FUnited States Pharmacopeia (USP) Reference Standard1134299SIG1134299-200MG-EA -
 Citalopram Related Compound GUnited States Pharmacopeia (USP) Reference Standard1134197SIG1134197-25MG-EA
Citalopram Related Compound GUnited States Pharmacopeia (USP) Reference Standard1134197SIG1134197-25MG-EA -
 Citalopram Related Compound HUnited States Pharmacopeia (USP) Reference Standard1134222SIG1134222-25MG-EA
Citalopram Related Compound HUnited States Pharmacopeia (USP) Reference Standard1134222SIG1134222-25MG-EA -
-
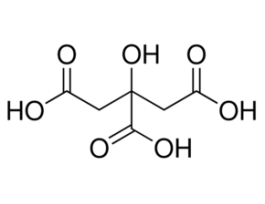
-

-
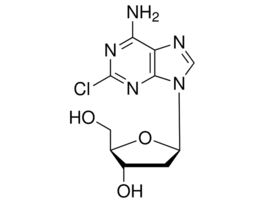 Cladribine for peak identificationEuropean Pharmacopoeia (EP) Reference StandardY0000609SIGY0000609-EA
Cladribine for peak identificationEuropean Pharmacopoeia (EP) Reference StandardY0000609SIGY0000609-EA -
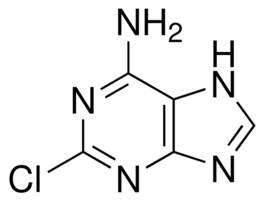
-
 Cladribine Related Compound AUnited States Pharmacopeia (USP) Reference Standard1134211SIG1134211-20MG-EA
Cladribine Related Compound AUnited States Pharmacopeia (USP) Reference Standard1134211SIG1134211-20MG-EA -

-
 Clarithromycin for peak identificationEuropean Pharmacopoeia (EP) Reference StandardY0000321SIGY0000321-EA
Clarithromycin for peak identificationEuropean Pharmacopoeia (EP) Reference StandardY0000321SIGY0000321-EA -
 Clarithromycin IdentityUnited States Pharmacopeia (USP) Reference Standard1134390SIG1134390-10MG-EA
Clarithromycin IdentityUnited States Pharmacopeia (USP) Reference Standard1134390SIG1134390-10MG-EA -
 Clarithromycin Impurity DUnited States Pharmacopeia (USP) Reference Standard1134619SIG1134619-10MG-EA
Clarithromycin Impurity DUnited States Pharmacopeia (USP) Reference Standard1134619SIG1134619-10MG-EA -
 Clarithromycin Impurity HUnited States Pharmacopeia (USP) Reference Standard1134620SIG1134620-10MG-EA
Clarithromycin Impurity HUnited States Pharmacopeia (USP) Reference Standard1134620SIG1134620-10MG-EA -
 Clarithromycin Impurity QUnited States Pharmacopeia (USP) Reference Standard1134631SIG1134631-10MG-EA
Clarithromycin Impurity QUnited States Pharmacopeia (USP) Reference Standard1134631SIG1134631-10MG-EA -
 Clarithromycin Impurity RUnited States Pharmacopeia (USP) Reference Standard1134642SIG1134642-10MG-EA
Clarithromycin Impurity RUnited States Pharmacopeia (USP) Reference Standard1134642SIG1134642-10MG-EA -

-

-

-
 Clazuril for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0000269SIGY0000269-EA
Clazuril for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0000269SIGY0000269-EA -
 Clemastine for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0001613SIGY0001613-EA
Clemastine for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0001613SIGY0001613-EA -
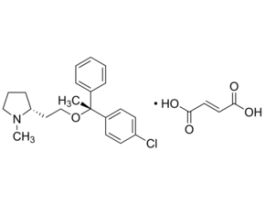
-

-

-
 Clenbuterol hydrochlorideUnited States Pharmacopeia (USP) Reference Standard1134674SIG1134674-100MG-EA
Clenbuterol hydrochlorideUnited States Pharmacopeia (USP) Reference Standard1134674SIG1134674-100MG-EA -

-
 Clenbuterol Related Compound BUnited States Pharmacopeia (USP) Reference Standard1134696SIG1134696-10MG-EA
Clenbuterol Related Compound BUnited States Pharmacopeia (USP) Reference Standard1134696SIG1134696-10MG-EA -
 Clidinium bromide Related Compound AUnited States Pharmacopeia (USP) Reference Standard1135021SIG1135021-250MG-EA
Clidinium bromide Related Compound AUnited States Pharmacopeia (USP) Reference Standard1135021SIG1135021-250MG-EA -

-

-

-
 Clindamycin hydrochlorideUnited States Pharmacopeia (USP) Reference Standard1136002SIG1136002-200MG-EA
Clindamycin hydrochlorideUnited States Pharmacopeia (USP) Reference Standard1136002SIG1136002-200MG-EA -
 Clindamycin palmitate hydrochlorideUnited States Pharmacopeia (USP) Reference Standard1137005SIG1137005-500MG-EA
Clindamycin palmitate hydrochlorideUnited States Pharmacopeia (USP) Reference Standard1137005SIG1137005-500MG-EA -

-

-

-
-
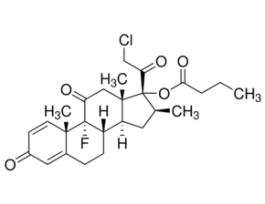
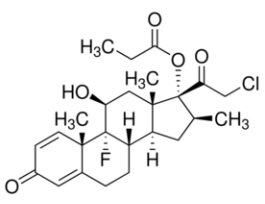
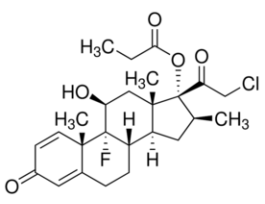 Clobetasol propionateUnited States Pharmacopeia (USP) Reference Standard1138405SIG1138405-200MG-EA
Clobetasol propionateUnited States Pharmacopeia (USP) Reference Standard1138405SIG1138405-200MG-EA -
-
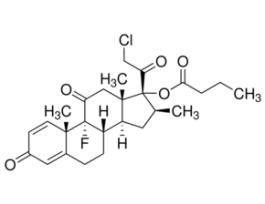
 Clocortolone pivalateUnited States Pharmacopeia (USP) Reference Standard1138507SIG1138507-200MG-EA
Clocortolone pivalateUnited States Pharmacopeia (USP) Reference Standard1138507SIG1138507-200MG-EA -
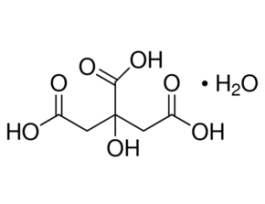
 Clodronate disodium tetrahydrateEuropean Pharmacopoeia (EP) Reference StandardY0000888SIGY0000888-EA
Clodronate disodium tetrahydrateEuropean Pharmacopoeia (EP) Reference StandardY0000888SIGY0000888-EA -

-
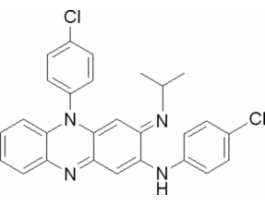
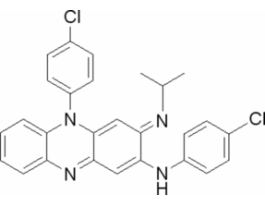 Clofazimine for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0000358SIGY0000358-EA
Clofazimine for system suitabilityEuropean Pharmacopoeia (EP) Reference StandardY0000358SIGY0000358-EA -

-

-

-
 Clomiphene Related Compound AUnited States Pharmacopeia (USP) Reference Standard1140101SIG1140101-100MG-EA
Clomiphene Related Compound AUnited States Pharmacopeia (USP) Reference Standard1140101SIG1140101-100MG-EA